Abstract
Mesenchymal stromal cells (MSC) are widely investigated for treating ARDS in Covid-19. Nonetheless, these efforts are overshadowed by studies predating the pandemic that mostly failed to show MSC efficacy in ARDS and recent disappointments with repurposed MSC products. Relying on years of MSC-related experience, Bonus BioGroup developed MesenCure: An enhanced allogeneic MSC therapy for Covid-19, professionalized by a unique combination of culture conditions and optimized in ARDS-relevant models.
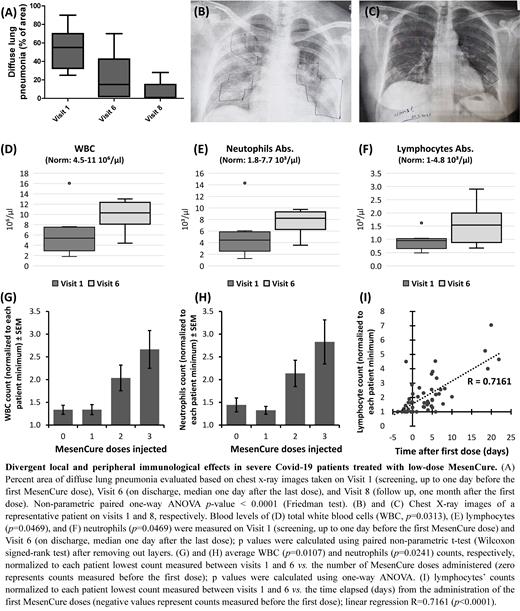
MesenCure is currently evaluated in a Phase II study in severe Covid-19 patients and administered (IV) in three doses (1.5M cells/kg, d1, d3, d5). A Phase I/II study on ten severe patients demonstrated a significant improvement in ARDS-related parameters following MesenCure treatment. Patients were discharged within one day (median) following treatment, requiring no respiratory support. Speedy recovery from local inflammation was observed in these patients, demonstrated by a rapid reduction in diffuse lung pneumonia, from 55% of the lung area to 15% within 5-6 days from the first dose (p<0.01, Fig. A-C). A corresponding drop in CRP was detected (p<0.01), which returned to normal. A multivariate regression analysis revealed that the reduction in CRP was mainly associated with the number of doses administered and not and the time elapsed since the first dose.
MesenCure efficacy may be attributable to the cells' de novo expression of the gene encoding for the IL-6 receptor, making them more responsive to inflammation than non-professionalized naïve MSC (NA-MSC); as well as >8-fold upregulation of the EDIL3 gene, encoding for an endogenous inhibitor of immune infiltration. A corresponding immunosuppressive effect of MesenCure MSCs was demonstrated in vitro, showing their ability to suppress T cells activation twice more effectively than NA-MSC. In this study, MesenCure inhibited the proliferation of primary CD4 T cells in a concentration-depended manner following non-specific activation. Over 98% inhibition was achieved in co-culture of 1:10 MSC-to-PBMC with an IC 50 of 6k MSC/200k PBMCs (r 2=1.00) compared to 12k NA-MSC/200k PBMCs (r 2=0.95). Comparable results were also obtained for CD8 T cells. Similarly, MesenCure inhibited ROS production by primary neutrophils remarkably fast and by up to 80% within less than 40 minutes following their activation (IC 50 = 19k MSC/200k neutrophils, r 2=1.00).
In addition to local immunosuppressive outcomes, a significant increase in blood leukocytes was observed in patients treated with MesenCure (p<0.05, Fig. D-F). Further analysis suggested that the increase in total WBCs and neutrophils was associated with the number of MesenCure doses administered (p<0.05, Fig. G-H). In contrast, the increase in lymphocytes was time-dependent (R=0.72, Fig. I).
The seemingly exclusively localized anti-inflammatory effects seen in severe patients treated with MesenCure were also observed in animal (murine) studies. An in vivo study in an acute lung injury model demonstrated a dose-dependent localized reduction in leukocyte counts in the lung fluids of animals treated with MesenCure (IV) using two dose levels. Relative to untreated animals, MesenCure reduced lung leukocyte counts by 35%-43% in animals treated with the low dose and by 62%-67% following high-dose MesenCure treatment (p<0.05). The leukocytes' clearance from the lungs was accompanied by a 41%-57% reduction in lung edema (p<0.05) following MesenCure treatment. Notably, NA-MSC did not achieve the same effect. Similar to our clinical findings, a significant increase was measured in neutrophil counts in animals treated with low-dose MesenCure (p<0.05), which decreased dramatically (p< 0.01) in animals treated with a four-times higher dose.
MesenCure is administered at a much lower dose compared to other MSC products administered at up to 10M cells/kg. Considering the increase in blood leukocytes measured in patients treated with low-dose MesenCure and comparable preclinical findings, our data suggest that low-dose MesenCure could elicit a potent local anti-inflammatory effect without suppressing, and even enhancing, peripheral immunity that is needed to fight the virus. Further research is inevitably required into the mechanism behind this phenomenon. However, our results indicate MesenCure's potential in relieving local inflammation while giving the patient a fighting chance against viremia.
Bronshtein: Bonus BioGroup: Current Employment. Ben David: Bonus BioGroup: Current Employment. Novak: Bonus BioGroup: Current Employment. Kivity: Bonus BioGroup: Current Employment. Meretzki: Bonus BioGroup: Current Employment. Rozen: Bonus BioGroup: Consultancy.